Striving for efficient phosphorus recycling solutions
Phosphorus recycling from sewage sludge: more fertilizer, fewer heavy metals
Phosphorus is essential as an agricultural fertilizer. Globally, this indispensable element is becoming increasingly scarce, putting an increasing focus on recovery where natural phosphate deposits are rare. Glatt Ingenieurtechnik GmbH has further developed its patented PHOS4green fertilizer production process so that sewage sludge ash from conurbations with high heavy metal contents can be used. Their removal means that fewer pollutants are released into the environment.
Glatt has expanded its PHOS4green process to facilitate the legally compliant recovery of valuable materials from sewage sludge ash contaminated with heavy metals
With sustainability now a global priority, the development of circular economies is top of mind throughout society, business and politics. The efficient use of resources, especially the recycling of waste products from material cycles, is becoming increasingly important. A good example is sewage sludge ash. Owing to its high phosphorus content, it offers promising approaches for recycling. Depending on the region (rural, metropolitan, etc.), source (municipal, industrial) and origin (animal waste, biomass, etc.), sewage sludge ash sometimes contains up to 10wt% of phosphorus mass, which is approximately 25wt% phosphorus pentoxide (P2O5). The European Parliament’s declaration on the inclusion of phosphorus in the list of critical raw materials in 2017 resulted in regulation that defined recycling strategies for this raw material.
Phosphorus: an essential nutrient
Phosphorus plays an essential role in life on Earth and is a key element in the energy metabolism and cell structure of most animals and plants; it’s required for growth, development and reproduction. Although humans absorb the chemical element through food, mineral fertilizers are used for crops. For this purpose, phosphorus is extracted from ore deposits in the form of various rock formations and processed into phosphoric acid. It’s the basis for various types of fertilizers. As the ore deposits are natural, the resulting fertilizers are not subject to any compliance regulations in terms of limit values for pollutants. This applies to high heavy metal concentration such as arsenic, cadmium and uranium (often meaning that the uranium content in drinking water is higher than it should be).
Owing to its non-biogenic origin, sewage sludge ash must have certain qualities to be processed into fertilizer granulates. This depends on the origin of the sewage sludge and the organic and inorganic contaminants. Limit values for these are defined in German law (German Fertilizer Regulation: DüMV) in Annex 2 DüMV and at the European level in Regulation EU-2019/1009 (Table I).
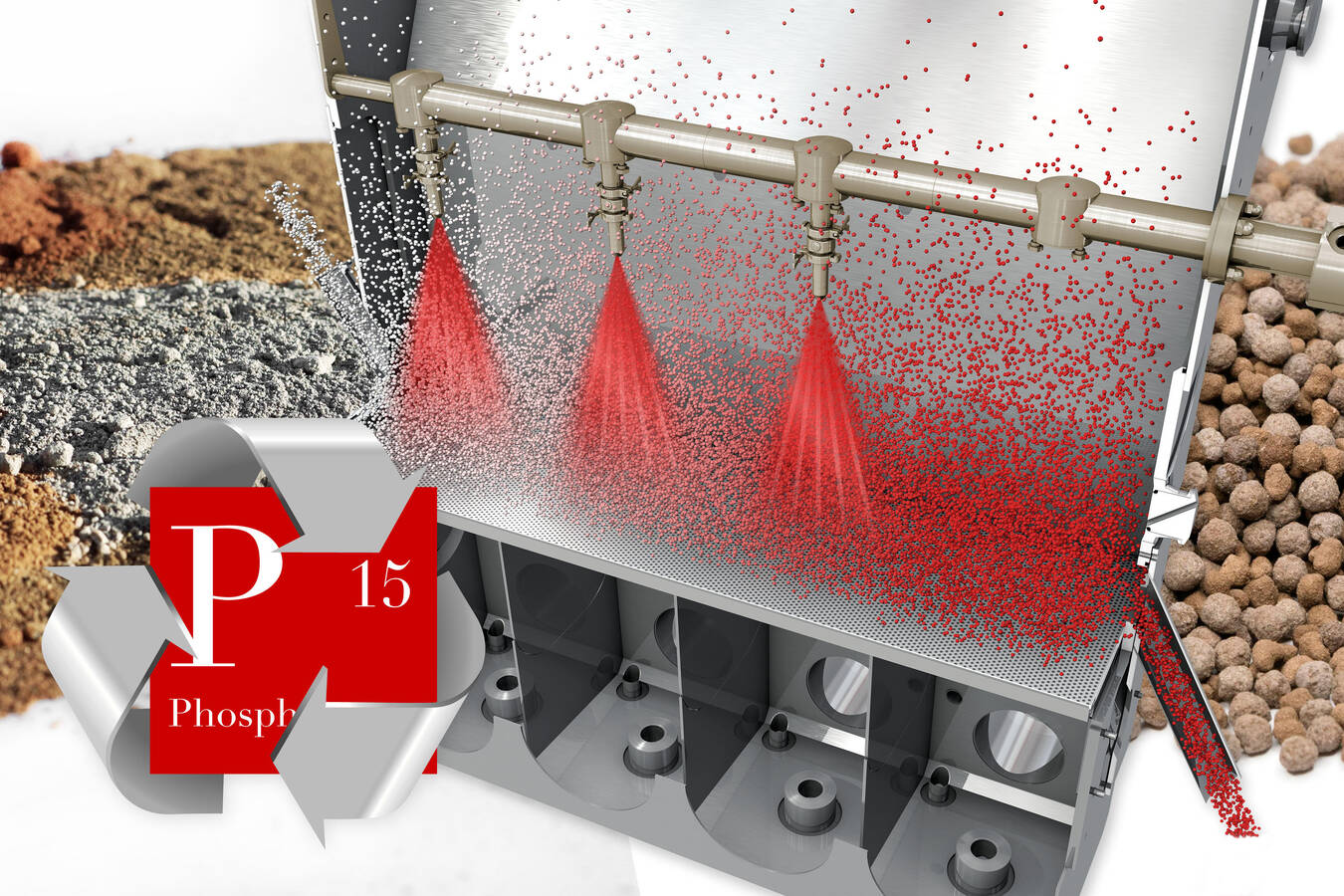
Glatt has accepted this challenge and developed the advanced PHOS4green technology. With this process, fertilizer granules with a precisely defined composition and specific particle properties (hardness, density and mineral availability) can be produced from sewage sludge ash. The particle size distribution can be freely adjusted in the range of 2–3 mm.
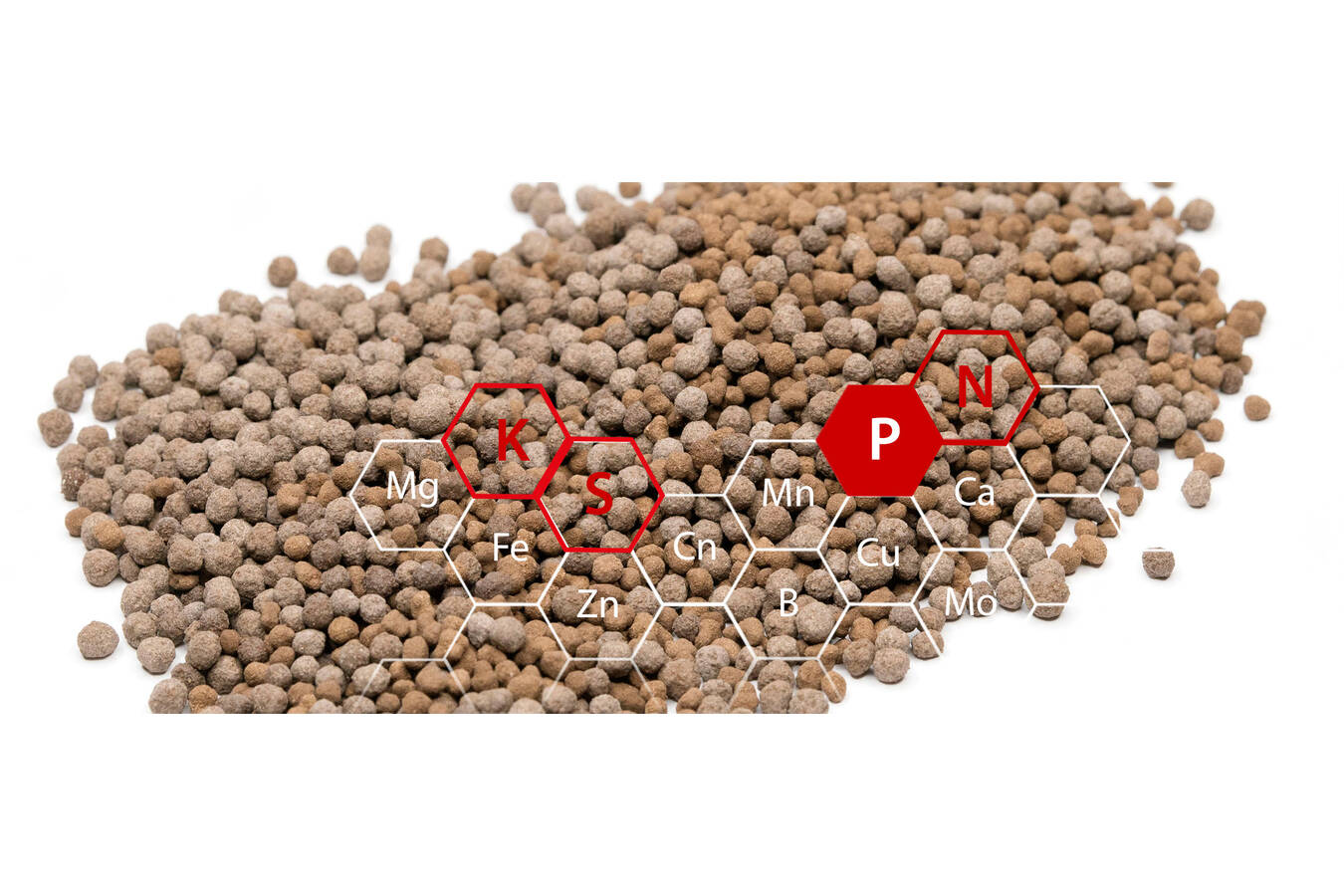
The basis for this continuous process is the processing of suspensions in fluidised bed and spouted bed apparatus. In addition, when processing ash with the PHOS4green process, macronutrients such as nitrogen, sulphur and phosphorus can be added to the spray suspension for the granulation process. In this way, customised multicomponent fertilizers can be produced from any ash.
Micronutrients — apart from boron and chloride — are heavy metals that can cause plant damage in high concentrations or enter the food chain and thus impair life processes and health. As part of the joint research project RePhoRM (Regional Phosphorus Recycling in the Rhine-Main Region), Glatt has developed various processes to reduce the concentration of heavy metals in the PHOS4green process depending on requirements and legal specifications.
Pushing the frontiers of sludge recycling
The collaborative RePhoRM project, founded by the German Federal Ministry of Education and Research (Grant No. 02WPR1545A-G), which is now being initiated after a successful trial phase, was set up to develop and implement a technological joint solution for phosphorus recycling in the eponymous area. It’s based on the local sludge incineration capacity and its potential expansion in the Frankfurt Rhine Main metropolitan region.
As such, the PHOS4green technology to produce fertilizer granules by removing heavy metals from (input) sewage sludge ash and implement the process on a large scale was further developed. Glatt also contributed its expertise to two subprojects: the planning and construction of a container plant at Industriepark Höchst and spray granulation studies on purified secondary phosphorus.
Three technological solutions for heavy metal removal
Depending on the required output and the quality of the input ash, the project identified three different ways to run the PHOS4green process.
(1) Phosphorus extraction
For ashes with levels of high heavy metal contamination (cadmium, lead and arsenic), washing with selected mineral acids was recommended to extract the phosphorus. Depending on the reaction time, poorly soluble heavy metal compounds remain as solids in the suspension. Shorter reaction times can minimise the dissolution of other heavy metals. Codissolved elements such as copper can be precipitated and removed by adding further additives. The separation of the liquid from the solid leads to a phosphoric acid that’s depleted of heavy metals, which is suitable to produce various fertilizer granulates like NPS, NPK, superphosphate analogue, P38 or P46 (Figure 1). The previously separated, heavy metal-enriched solids are produced as waste.
Example application: An ash (composition listed in Table II) was used to assess the applicability of the process. This was treated with two formulations (R1 and R2). The requirement was to extract at least 80% of the phosphorus with the lowest possible redissolution of the heavy metals. The course of the reaction was monitored for 60 minutes.
Figure 2 shows that, despite the longer period and the higher solids content of the suspension, formulation R2 delivers a constant redissolution rate of the phosphorus bound in the ash. The redissolution of heavy metals is lower for formulation R1 compared with R2 (apart from arsenic). See Table III.
Additional heavy metal removal by precipitating the heavy metals (ideally as sulphides) directly from the suspension would further reduce the concentration. This means that high removal rates can also be achieved for arsenic and copper.
(2) Heavy metal extraction
For fertilizer manufacturers, it’s advisable to produce as little waste as possible and use all the ash. Compared with phosphorus extraction, the focus here is on the selective removal of heavy metals. This can be achieved by using selected mineral acids and mixtures under oxidative conditions, longer reaction times and higher pH values. Figure 3 shows how heavy metals dissolve out of the mineral and concentrate in the solution with time.
As a result, heavy metals are selectively kept in solution while phosphorus remains predominantly in the solid (redissolution rates <40%). The suspension is filtered and the heavy metals remaining in the solution are precipitated. The degradation rate of the heavy metals is defined by the redissolution rate from the ash and the precipitation rate from the extract. After separation, the depleted fractions are recombined and fertilizer granules with the desired composition are obtained by adding further additives. The depletion rates are lower compared with the first option, which is why this process is suitable for ash with low heavy metal concentrations. The precipitated heavy metals must be disposed of as waste. Example application: An ash (composition listed in Table IV) was used to assess the applicability of the process. This was treated with four different formulations (F1–F4) for up to 60 minutes. The redissolution rate is highly dependent on the mineral acid or mineral acid mixture used. It was found that a reaction time of 40 minutes results in sufficiently high extraction rates of the heavy metals (Figure 4).
The heavy metals were precipitated as sulphides after separation from the liquid phase (Figure 5). The degradation rates depend on the formulation used. This is because of the redissolution rate of the heavy metals from the ash, but also the precipitation rate from the liquid phase (particularly arsenic). However, if the pH value of the liquid phase is increased too much, phosphorus precipitates as a poorly soluble compound of iron, aluminium and calcium, which should be avoided. In general, an increase in the precipitant content also results in higher heavy metal degradation rates. The process can therefore be flexibly adapted to the respective conditions.
(3) Combination process
The second process can be extended by first extracting the phosphorus from the ash and precipitating the codissolved heavy metals. The resulting heavy metal-depleted phosphoric acid is separated from the solid. A second process step extracts the heavy metals from the solid matrix by oxidation. After the separation step, the heavy metals are precipitated from the solution again. As a large proportion of the phosphorus has already been extracted in the first step, only a small proportion of phosphates precipitates when the pH value is raised. Most of the heavy metals can be completely removed at pH values above 5. This is a major advantage when it comes to removing high levels of nickel and zinc. By combining the solid and liquid phases and adding further additives, fertilizers of the desired composition can be produced. The precipitated heavy metals are waste but can be purified and recovered.
PHOS4green: quality derived from flexibility
As explained above, the introduction of phosphorus recycling strategies from sewage sludge ash is becoming increasingly important, particularly considering EU regulations and the German Fertilizer Ordinance. The challenge is to remove heavy metals and comply with limit values.
As an initial priority, the focus is on establishing local supply chains and strengthening the circular economy. Spray granulation is used to produce fertilizer granules from sewage sludge ash. PHOS4green technology makes it possible to individually adjust the composition and particle properties of those granules.
If the process is expanded to include selective heavy metal removal (using mineral acids), less waste is produced because all the ash is utilised. This process variant is suitable for ash with low heavy metal concentrations.
If double extraction is added to the basic process, both phosphorus and heavy metals can be extracted in two steps. In the second step, heavy metals such as nickel and zinc can be removed effectively.
Overall, Glatt strives for efficient recycling solutions to offer fertilizer manufacturers high-quality products while minimising their environmental impact. The company’s innovative technologies help to overcome the challenges of phosphorus recycling from sewage sludge ash and contribute to a sustainable circular economy.
author: Dr. Johannes Buchheim, Glatt Ingenieurtechnik
originally published in the professional magazine ‘World Fertilizer’, issue May/June 2024, Palladian Publications Ltd